Clinical Lipidomics
Please find information and connection details about the regular CLIG meeting here:
The main goal of this interest group (IG) is to help with the translation of lipidomic research from academia to clinical laboratories for diagnostic and therapeutic purposes. The first task is the identification of bottlenecks in this process, and how to overcome possible difficulties in clinical translation of lipidomic technology. The typical clinical laboratory processes a large number of samples daily, therefore any analytical method considered for the translation has to be robust, simple, cost acceptable, preferably automated, and validated in line with guidelines for bioanalytical validation. All clinical tests have to be compliant with regulatory requirements, such as EU IVDR addressing the entire lifecycle of a product. However, we recognize that the measurement of over hundred analytes in a single assay may require some new considerations regarding the guidelines and quality assurance programs.
The clinical analysis is typically concerned with the analysis of body fluids. The most frequently analyzed body fluids are serum, plasma, and urine. Therefore, the clinical lipidomics should be also aligned with the established clinical workflows for the sample collection. Other types of body fluids or tissues (e.g., biopsies) may be used for dedicated applications. Preanalytics need to be feasible for clinical chemistry workflows.
The Clinical lipidomics IG should lay out basic requirements for the method translation to the real clinical practice to help with the implementation of lipidomic laboratory tests. The use of molar concentrations is advised in line with the established clinical practice, where the reference intervals of molar concentrations should be known for healthy population, for example as for cholesterol. This IG should work in a close cooperation with Reference materials IG, regulatory authorities responsible for the approval of laboratory tests, and experts from clinical laboratories. Many analytical configurations are used for the research quantitation of lipids, but we need to narrow a range of applicable workflows in line with current technologies used in clinical laboratories.
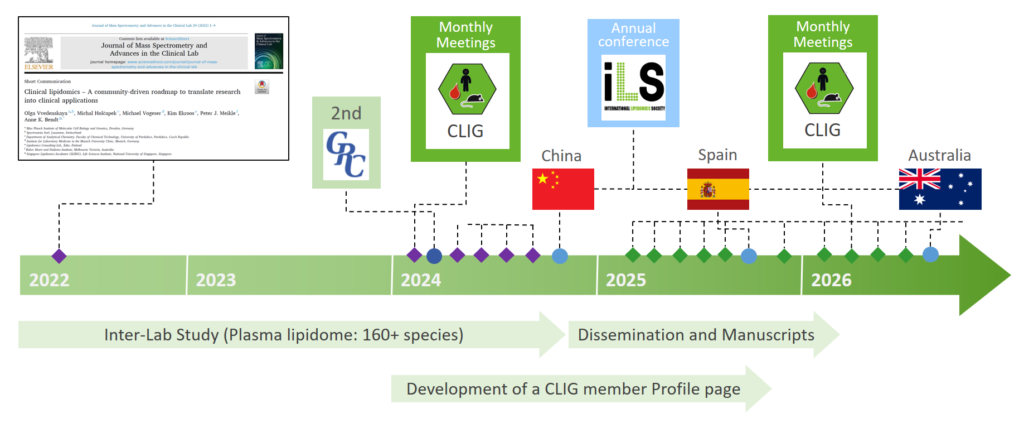
Steering committee:
Michal Holčapek, Czech Republic
Michael Vogeser, Germany
Olya Vvedenskaya, Germany
Regular Meetings
IG Clinical Lipidomics Regular Meeting
The Clinical Lipidomics Interest Group invites you to its regular meetings on the first Tuesday of every month at the following times: We want to
Interest Group News
AI-Generated Summary of the Regular CLIC Meeting held on 1st April 2025
IG Clinical Lipidomics Publication
IG Clinical Lipidomics Webinar: How to translate the lipidomic analytical method into real clinical practice?
Date and time: Tuesday November 3, 2020, 14:00 – 17:00 (Central European Time, CET) Organization: Clinical lipidomics interest group in International Lipidomics Society, free to
CLIG Members
External source embedded from Airtable.com, with permission of the featured members.







