Reference materials and biological reference ranges
The main aim of this interest group is to aid the process towards determination of the biological variation of lipids. Knowledge of the upper and lower concentrations boundaries of lipids forms the basis for scientific advances as well as technology translation in the field of lipidomics.
A reference material is a homogeneous pool of unprocessed matrix representative of the study matrix. In a lipidomic experiment it is processed in identical fashion to the study samples and analyzed in regular intervals to those. Design, production, storage and dissemination of reference materials is a complicated endeavor typically supported by dedicated public sector institutes and/or industry. This interest group will also be a partner for deliberations related to the development of more stable isotope standards and combination of such standards, a second line of reference materials critical in mass spectrometry of lipids.
Shared reference materials are an effective way to harmonise experiments across mass spectrometry-based platforms and among laboratories. Therefore, it is expected that our activities will produce additional benefits and serve other interest groups within ILS and beyond.
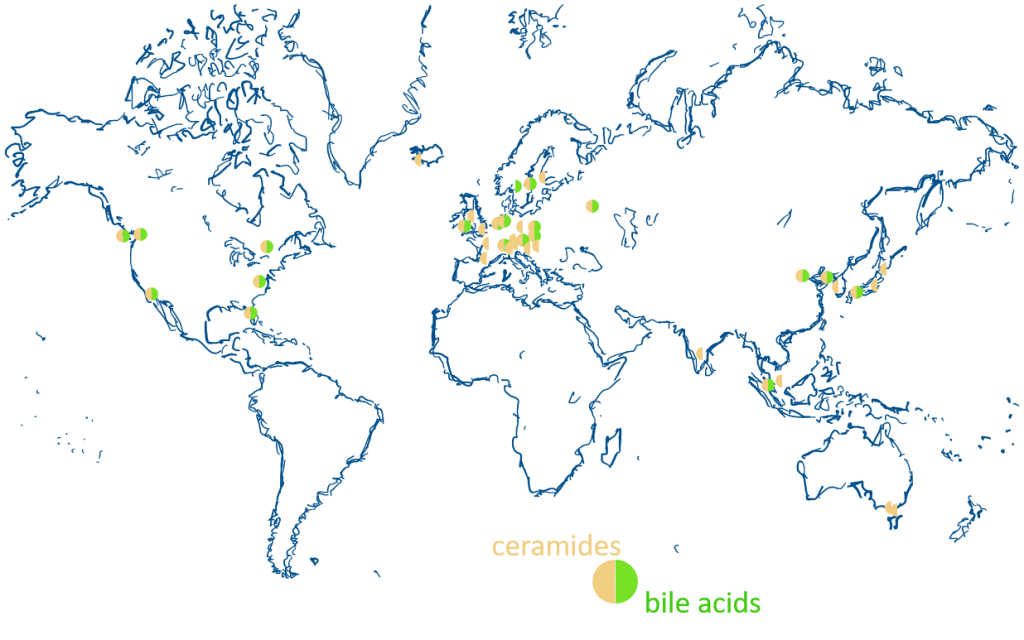
Two workshops held in Singapore in April 2017 and November 2018 paved the way for this community-driven effort in order to develop accepted guidelines for mass spectrometry-based lipidomics of human blood plasma. On 2019, a carousel of international meetings was utilised to further specify details for ‘ring-trials’ among volunteering participant laboratories and partners. To reduce complexity, while maintaining strict focus on human plasma/serum, a staged approach was adopted. Currently, small groups (i.e. coordination groups) have been organising efforts for ceramides and bile acids. The overarching aims are to first achieve technical replication in a global network (phase I). Subsequently, we aim to use this basis to determine biological reference ranges in different human populations (phase II).
Steering committee:
Ceramide ring trial reference protocols available
The protocols for the Ceramide ring trial organized and conducted by the Interest Group Reference Materials and Biological Reference Ranges are now available for download:
Ceramides
The inaugural class of lipids have been shortlisted based on technical feasibility (analyte stability, available standards, coherence of different methods) and potential utility (clinical relevance). Currently, 40 laboratories are using their preferred analytical method and/or a ‘standard’ protocol. Packages containing aliquots of NIST1950 and three additional NIST pooled plasma samples from different sources, as well as appropriately formulated mixtures of ceramide standards, were distributed either directly from Avanti polar lipids or via the Singapore Lipidomics Incubator (SLING). The goal for phase I is to report quantification of 4 distinct ceramide species (Cer d18:1/16:0, Cer d18:1/18:0, Cer d18:1/24:0, Cer d18:1/24:1) in the pooled plasma samples.
Coordination team:
Federico Torta, Singapore
John Bowden, USA
Kim Ekroos, Finland
Nils Hoffmann, Germany
Robert Ahrends, Austria
Bile Acids
Of broad clinical interest and potential for extension of existing panel towards less well characterised species (including secondary bile acids). Current network of 22 laboratories will be further extended also by the recently established coordination team.
Coordination team:
Amaury Cazenave-Gassiot, Singapore
Gerhard Liebisch, Germany
Bill Griffiths, Wales
Hans-Frieder Schött, Singapore
Alex Dickens, Finland
Tuulia Hyotylainen, Sweden









